ORGANIC CHEMISTRY
|
|||
แรงระหว่างโมเลกุล (Intermolecular forces)
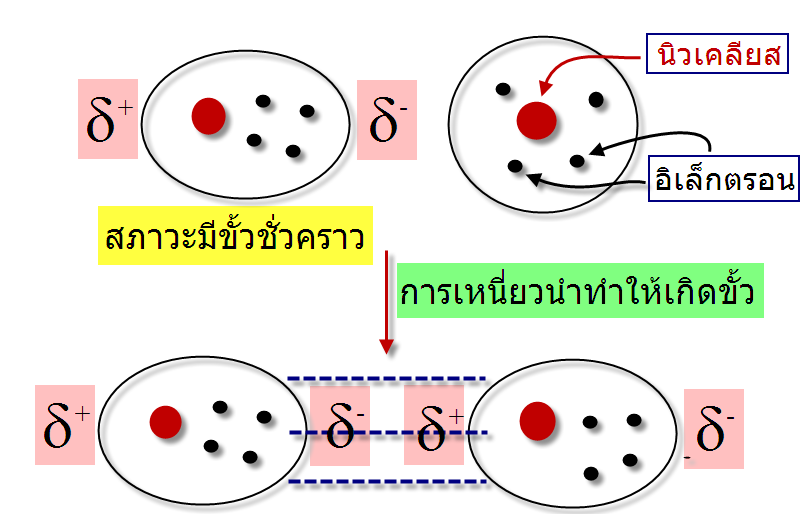
ในทางเคมีการศึกษาเรื่องแรงระหว่างโมเลกุลมีประโยชน์ในการทำนายจุดเดือดและจุดหลอมเหลวของสารประกอบอินทรีย์ จัดเป็นแรงที่กระทำระหว่างโมเลกุลหรือระหว่างหมูฟังก์ชันของสารประกอบแบ่งได้เป็นสองประเภทคือ แรงแวนเดอร์วาลล์(Van der Waals forces) และพันธะโฮโดรเจน (Hydrogen bonding) พบว่าแรงระหว่างโมเลกุลมีความแข็งแรงน้อยกว่าพันธะภายในโมเลกุล เช่น พันธะไอโอนิก และพันธะโคเวเลนซ์ แรงแวนเดอวาลล์ (Van der Waals forces) จัดเป็นแรงระหว่างโมเลกุลมีความแข็งแรงน้อยกว่าพันธะไฮโดรเจน(Hydrogen bonding)ซึ่งแบ่งเป็นสามประเภทคือ แรงลอนดอน (London dispersion forces) แรงดึงดูดระหว่างขั้ว(Dipole-dipole forces)และแรงดึงดูดไอออนกับขั้ว(Ion-dipole forces) -แรงลอนดอน (London โดยปกติโมเลกุลที่ไม่มีขั้วจะประพฤติตัวเป็นกลางในบางครั้งอิเล็กตรอนเคลื่อนที่ไปรวมอยู่ด้านใดด้านหนึ่งของอะตอมหรือโมเลกุลทำให้เกิดสภาพขั้วชั่วคราวขึ้น โดยตำแหน่งที่อิเล็กตรอนเคลื่อนที่ไปรวมกันจะเกิดสภาพที่เป็นประจุลบ (d-) และตำแหน่งที่ไม่มีอิเล็กตรอนจะเกิดสภาพประจุที่เป็นบวก (d+) โมเลกุลที่มีสภาพขั้วแบบชั่วคราวสามารถเหนี่ยวนำทำให้โมเลกุลในสภาวะปกติมีสภาพขั้วโดยที่ตำแหน่งที่มีอิเล็กตรอนหนาแน่นมีประจุเป็นลบจะไปผลักอิเล็กตรอนของอีกโมเลกุลให้ไปอยู่ด้านตรงข้ามทำให้เกิดอีกโมเลกุลมีสภาวะขั้วชั่วคราวอีกโมเลกุล ทำให้โมเลกุลทั้งสองมีประจุบวกและลบเรียกว่า Polarizability และเกิดแรงดึงดูดระหว่างประจุบวกและลบ ซึ่งแรงดึงดูดที่เกิดขึ้นดังลักษณะนี้เราเรียกว่า แรงลอนดอน (London dispersion force)
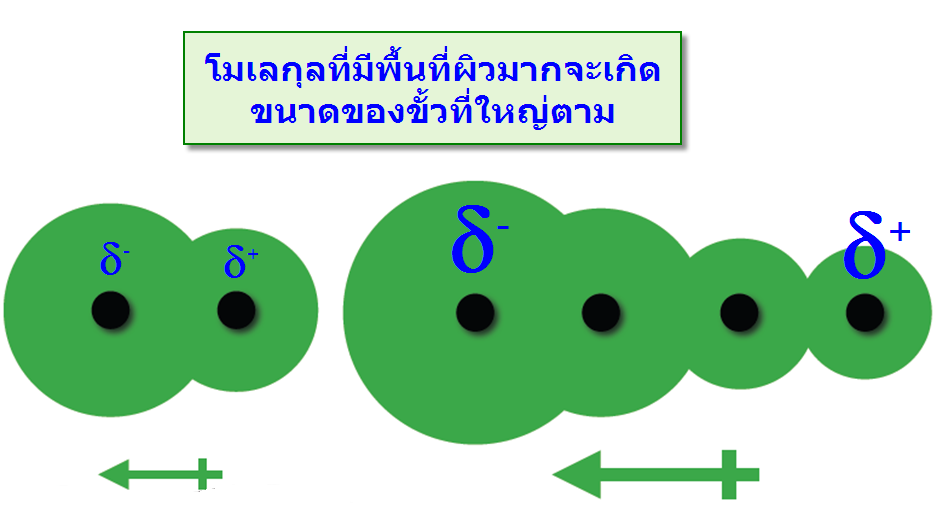
แรงที่เกิดขึ้นนี้เกิดจากการที่อิเล็กตรอนเคลื่อนที่ไปรวมอยู่ด้านใดด้านหนึ่งของโมเลกุลทำให้เกิดสภาพขั้วชั่วคราวขึ้น และไม่สามารถเกิดขึ้นแบบถาวรจึงมีความแข็งแรงจึงน้อยมาก พบว่าเมื่อโมเลกุลหรืออะตอมมีขนาดใหญ่ขึ้นจะมีความสามารถทำให้เกิดขั้ว -พื้นที่ผิวของโมเลกุล : โมเลกุลที่มีพื้นที่ผิวมากจะทำให้ขนาดของประจุมีขนาดใหญ่ตาม เมื่อเปรียบเทียบสถานะของ Neopentaneและ n-Pentaneพบว่าที่อุณหภูมิห้อง
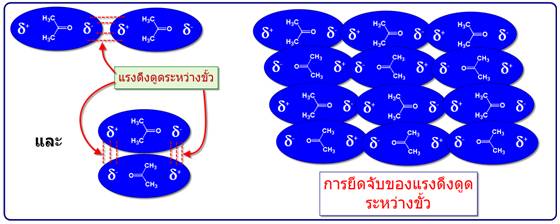
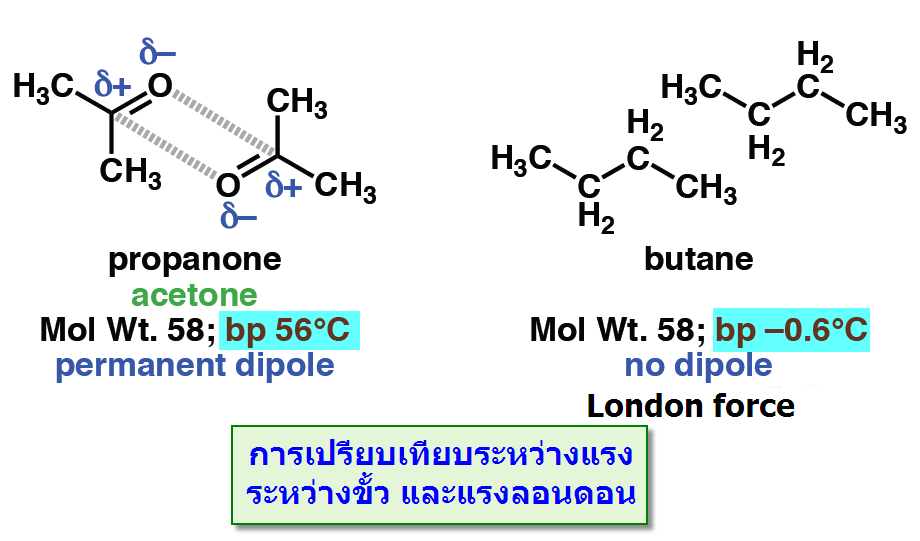
-แรงดึงดูดระหว่างขั้ว (Dipole -dipole forces) จัดเป็นแรงที่เกิดจากขั้วถาวรดึงดูดกันเองระหว่างขั้วบวกและขั้วลบซึ่งดึงดูดได้สองแบบดังตัวอย่างของอะซิโตน
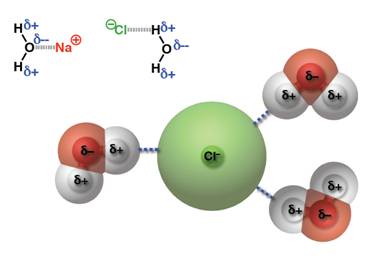
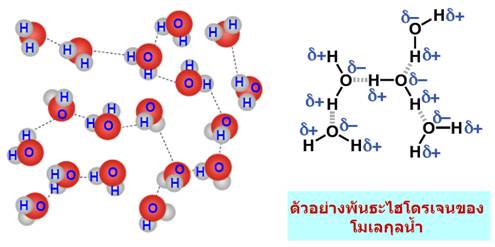
เมื่อนำมาเปรียบเทียบระหว่างแรงดึงดูดระหว่างขั้ว(Dipole -dipole forces) กับ แรงลอนดอน (London - แรงดึงดูดระหว่างไอออนกับขั้ว(Ion-dipole -พันธะโฮโดรเจน(Hydrogen bonding) จัดเป็นพันธะที่แข็งแรงมากที่สุดในพันธะระหว่างโมเลกุลเกิดระหว่างอะตอมของ H และ อะตอมที่มีค่า EN สูง F, O, N
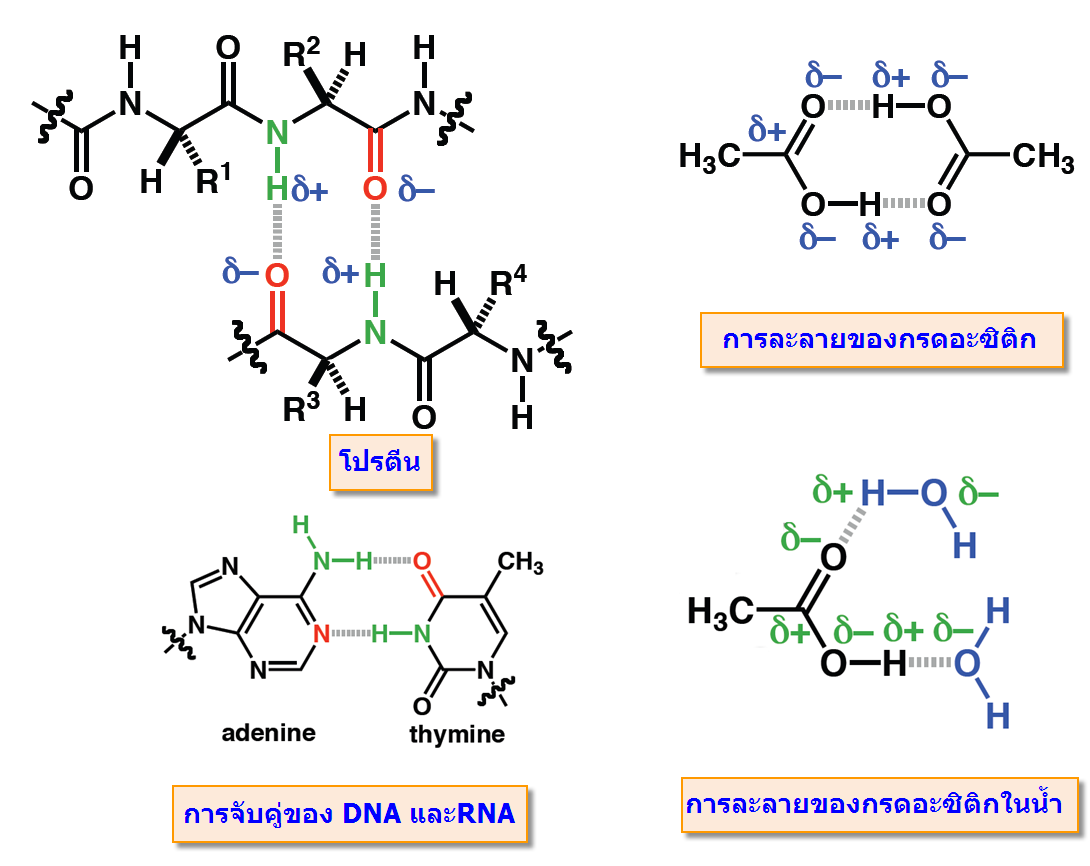
พันธะโฮโดรเจน(Hydrogen bonding) มีบทบาทสำคัญกับขบวนการที่สำคัญของสิ่งมีชีวิต ไม่ว่าเป็นการจับกันระหว่างโมเลกุลของโปรตีน และการจับเข้าคู่กับของ DNAและRNA
|
Organic_D
 ผู้ติดตามบล็อก : 2 คน [?] ผู้ติดตามบล็อก : 2 คน [?] Basic Chemistry,Organic Chemistry Group Blog Link |
||
| Pantip.com | PantipMarket.com | Pantown.com | © 2004 BlogGang.com allrights reserved. | |||